Stem cells for scar treatment are explored due to their regenerative properties. Wound healing is a routine process to repair an injury. However, it is a complex process involving different cells and phases. The imbalance in the process can either lead to scar or fibrosis. In both cases, tissue loses its original structure and function. While it has modest implications in subcutaneous tissue, its presence in internal organs can severely impact its function. The scar formed in the tissue is more susceptible to injury.
Therefore, research is focusing on a treatment that can drive scarless healing. In the last decades, regenerative medicine has caused a shift in the therapeutic approach from merely treating the disorder to stimulating tissue repair. Stem cell therapy has been at the forefront of this approach. In this blog, let’s explore how wound healing occurs and whether Stem Cells Can Heal Scar Tissue.
Wound Healing Process
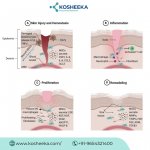
In case of any injury, the wound repair process begins. It comprises three different cell types, including endothelial cells, leukocytes, and fibroblasts. The repair proceeds in three overlapping phases.
Inflammatory Phase
Blood loss and capillary damage are indicators of the onset of tissue injury. This triggers the platelets to form a fibrin clot to prevent blood loss and the entry of pathogens. Platelets also release chemokines to recruit immune cells to the injury site to engulf pathogens. Neutrophils are the first to arrive, followed by macrophages. Moreover, injury creates a hypoxic environment that induces the secretion of growth factors, which promote the influx of fibroblasts and endothelial cells.
Proliferative Phase
This phase engages in active healing. Endothelial cells promote angiogenesis, restoring the blood perfusion. The oxygen and nutrient supply stimulate cell proliferation. Fibroblasts secrete extracellular matrix (ECM) proteins. The tissue repair with reinstated vessel and ECM is referred to as granulation tissue. It differs from the original tissue by the presence of collagen III and the lack of elastin.
Remodeling Phase
Fibroblasts differentiate into myofibroblasts in this phase. Myofibroblasts remodel the matrix by regulating the balance between matrix metalloproteinase (MMPs) and their inhibitors (TIMPs). These cells replace collagen III with collagen I, a constituent of the original tissue ECM before damage. Myofibroblasts also express α-smooth muscle actin (α-SMA), which confers contractility to these cells and aids in wound contraction and closure.
Scar Tissue Formation
An abnormal wound healing process results in a scar. The key dysregulation occurs in the remodeling phase. While excessive collagenase activity forms non-healing ulcers, decreased collagenase activity leads to hypertrophic scarring or keloids. Accumulation of ECM proteins in higher amounts results in organ fibrosis, which is involved in liver failure, pulmonary fibrosis, kidney failure, etc. The imbalance between MMPs and TIMPs is held responsible for irregular ECM deposition. However, the mechanisms behind it are not clear yet. Unlike healthy tissue, scar tissue lacks the flexibility, strength, and standard cellular architecture of the original tissue.
Can Stem Cells Reduce Scar Tissue?
Stem cells have regenerative potential that can benefit tissue repair. Therefore, the mechanisms behind Stem Cell Treatment of Scar Tissue, particularly with mesenchymal stem cells (MSCs), have been investigated.
Immunomodulation: Starting from the first phase, MSCs can regulate inflammation. They increase the expression of prostaglandin E2 (PGE2) and the activity of cyclooxygenase-2 (COX2). PGE2 decreases the level of IL2, a key mediator of T cell activation and proliferation. In response to PGE2, T cells and macrophages express IL10, an anti-inflammatory cytokine. Therefore, MSCs control chronic inflammation that aggravates scar formation.
Angiogenesis: MSCs secrete VEGF-A and FGF to promote the migration, proliferation, and differentiation of endothelial cells. This results in the formation and stabilization of the vascular network.
Anti-fibrosis: MSCs also release IL10 and hepatocyte growth factor (HGF) that attenuate fibrosis. HGF increases the expression of several MMPs that promote ECM turnover. It also inhibits myofibroblast formation, thereby diminishing pro-fibrotic functions.
In addition to MSCs, tissue-derived stem cells such as lung progenitors, cardiac stem cells, and liver progenitors have also been employed for scar reduction. The answer to “can stem cells remove the scar” is no. Stem Cell Treatment can reduce the scar, but there have been no reports of complete scar removal.
Future Perspectives
Any aberration in the wound healing results in scar formation. Opposed to the popular belief, scars do not just appear on subcutaneous tissue but also impact organ repair. With multiple phases and cells involved in the process, the therapeutic development is challenging. Stem cells possess the ability for effective scarless repair by acting on all the phases. The clinical trials have evaluated the therapy, showing significant effects in cardiac, pulmonary, and liver diseases.
More strategies are under investigation to improve the therapeutic efficiency of stem cell treatment. For instance, seeding stem cells on scaffolds enhances tissue regeneration in subcutaneous healing. The addition of growth factors to these scaffolds further increases the stem cell function. Large-scale clinical trials will soon avail the therapy in hospital settings. Kosheeka delivers the premium-quality MSCs from different sources after robust testing and standard protocols to ensure uniformity in each batch and each experiment.
Therefore, research is focusing on a treatment that can drive scarless healing. In the last decades, regenerative medicine has caused a shift in the therapeutic approach from merely treating the disorder to stimulating tissue repair. Stem cell therapy has been at the forefront of this approach. In this blog, let’s explore how wound healing occurs and whether Stem Cells Can Heal Scar Tissue.
Wound Healing Process
In case of any injury, the wound repair process begins. It comprises three different cell types, including endothelial cells, leukocytes, and fibroblasts. The repair proceeds in three overlapping phases.
Inflammatory Phase
Blood loss and capillary damage are indicators of the onset of tissue injury. This triggers the platelets to form a fibrin clot to prevent blood loss and the entry of pathogens. Platelets also release chemokines to recruit immune cells to the injury site to engulf pathogens. Neutrophils are the first to arrive, followed by macrophages. Moreover, injury creates a hypoxic environment that induces the secretion of growth factors, which promote the influx of fibroblasts and endothelial cells.
Proliferative Phase
This phase engages in active healing. Endothelial cells promote angiogenesis, restoring the blood perfusion. The oxygen and nutrient supply stimulate cell proliferation. Fibroblasts secrete extracellular matrix (ECM) proteins. The tissue repair with reinstated vessel and ECM is referred to as granulation tissue. It differs from the original tissue by the presence of collagen III and the lack of elastin.
Remodeling Phase
Fibroblasts differentiate into myofibroblasts in this phase. Myofibroblasts remodel the matrix by regulating the balance between matrix metalloproteinase (MMPs) and their inhibitors (TIMPs). These cells replace collagen III with collagen I, a constituent of the original tissue ECM before damage. Myofibroblasts also express α-smooth muscle actin (α-SMA), which confers contractility to these cells and aids in wound contraction and closure.
Scar Tissue Formation
An abnormal wound healing process results in a scar. The key dysregulation occurs in the remodeling phase. While excessive collagenase activity forms non-healing ulcers, decreased collagenase activity leads to hypertrophic scarring or keloids. Accumulation of ECM proteins in higher amounts results in organ fibrosis, which is involved in liver failure, pulmonary fibrosis, kidney failure, etc. The imbalance between MMPs and TIMPs is held responsible for irregular ECM deposition. However, the mechanisms behind it are not clear yet. Unlike healthy tissue, scar tissue lacks the flexibility, strength, and standard cellular architecture of the original tissue.
Can Stem Cells Reduce Scar Tissue?
Stem cells have regenerative potential that can benefit tissue repair. Therefore, the mechanisms behind Stem Cell Treatment of Scar Tissue, particularly with mesenchymal stem cells (MSCs), have been investigated.
Immunomodulation: Starting from the first phase, MSCs can regulate inflammation. They increase the expression of prostaglandin E2 (PGE2) and the activity of cyclooxygenase-2 (COX2). PGE2 decreases the level of IL2, a key mediator of T cell activation and proliferation. In response to PGE2, T cells and macrophages express IL10, an anti-inflammatory cytokine. Therefore, MSCs control chronic inflammation that aggravates scar formation.
Angiogenesis: MSCs secrete VEGF-A and FGF to promote the migration, proliferation, and differentiation of endothelial cells. This results in the formation and stabilization of the vascular network.
Anti-fibrosis: MSCs also release IL10 and hepatocyte growth factor (HGF) that attenuate fibrosis. HGF increases the expression of several MMPs that promote ECM turnover. It also inhibits myofibroblast formation, thereby diminishing pro-fibrotic functions.
In addition to MSCs, tissue-derived stem cells such as lung progenitors, cardiac stem cells, and liver progenitors have also been employed for scar reduction. The answer to “can stem cells remove the scar” is no. Stem Cell Treatment can reduce the scar, but there have been no reports of complete scar removal.
Future Perspectives
Any aberration in the wound healing results in scar formation. Opposed to the popular belief, scars do not just appear on subcutaneous tissue but also impact organ repair. With multiple phases and cells involved in the process, the therapeutic development is challenging. Stem cells possess the ability for effective scarless repair by acting on all the phases. The clinical trials have evaluated the therapy, showing significant effects in cardiac, pulmonary, and liver diseases.
More strategies are under investigation to improve the therapeutic efficiency of stem cell treatment. For instance, seeding stem cells on scaffolds enhances tissue regeneration in subcutaneous healing. The addition of growth factors to these scaffolds further increases the stem cell function. Large-scale clinical trials will soon avail the therapy in hospital settings. Kosheeka delivers the premium-quality MSCs from different sources after robust testing and standard protocols to ensure uniformity in each batch and each experiment.